A new draft guidance on rare disease trials may help sponsors ease the tensions between the need for robust, scientifically sound data and the small patient populations that they’re hoping to treat.
The guidance offers sponsors advice on filling the gap by using such tools as natural histories, surrogate biomarkers, external controls and early randomization but perhaps most importantly, offers a new section on safety.
Lindsay McNair, chief medical officer of WCG Clinical, says the guidance “synthesizes and updates information for the sponsors and researchers working in these therapeutic areas and addresses some of the issues that are frequently challenging.”
“I would say that the challenge in developing new therapies for rare diseases is that it often involves a lot of groundbreaking with little regulatory precedent and challenges in the study design, such as determining what the appropriate endpoints will be,” McNair says.
In the new section on drug safety, the guidance encourages sponsors to come up with their own ideas for rare disease safety data, but also offers some suggestions on common approaches.
Drug sponsors should consider natural histories, trial eligibility, dose selection, computer arms or auxiliary safety cohorts as means to ensure that they’re giving regulators the best — and most — possible safety data for rare disease treatments, the draft says.
Canvassing possible approaches to expanded safety data, the agency says of each:
- Natural histories can “help distinguish drug-related adverse effects from underlying disease manifestations;”
- Trial eligibility: sponsors should “consider enrichment strategies to decrease heterogeneity and to enhance the ability of the clinical trial to demonstrate a potential treatment effect;”
- Attention to dose selection is important to make sure that patients don’t stop taking medicines because their doses are too low or high; and
- Auxiliary cohorts can include a trial protocol with a parallel safety cohort (alongside an efficacy trial), a trial that gives patients drugs under expanded access or gathers relevant data from other sources, such as trials using the drug for another purpose or studies of similar drugs.
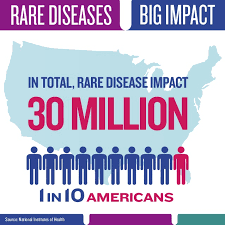
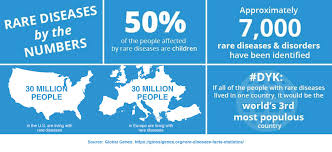
When considering a rare disease drug’s feasibility — which also can be tough, given the small populations involved — sponsors should include all the disease’s permutations through different life stages of its patients.
“Sponsors also should determine prevalence estimates for all countries in which trial sites are being considered,” the document states. “Sponsors should provide the individual sources of current published prevalence estimates, rather than calculated averages, because published prevalence estimates can vary widely depending on study details … country or region, and advances in diagnostics and treatment over time.”
You can read the FDA’s new draft guidance here: https://bit.ly/2FAUUr6.

Drug sponsors should consider natural histories, trial eligibility, dose selection, computer arms or auxiliary safety cohorts as means to ensure that they’re giving regulators the best — and most — possible safety data for rare disease treatments, the draft says.