Effectively communicating a trial’s benefits and risks to prospective subjects requires an understanding of how different types of patients process information.
Trials with high benefit and low risk clearly are easiest to communicate to patients. At the other end of the scale, however, high-risk, low-benefit trials require an understanding of patients’ needs and attitudes.
The Medical Device Innovation Consortium’s (MDIC) recent Patient-Centered Benefit-Risk Project Report offers guidance on how to help patients make trial participation decisions and stresses that the key to clear communication is knowing how — and what — patients think.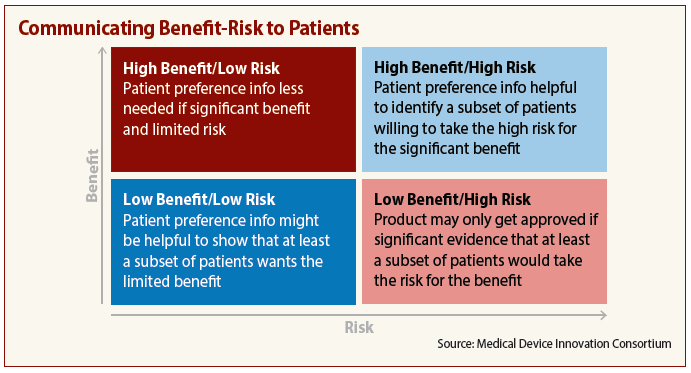
Factors to consider when designing patient communication, MDIC says, include the following:
- Patients age 65 or older often need input from a trusted party, so it can be helpful to work with the doctors they know to explain what could happen in a trial;
- Patients with a higher verbal aptitude understand benefit-risk analysis presented in words, but patients with higher graphic literacy better comprehend data presented in charts and graphs;
- Patients with a lower ability to comprehend numbers do not benefit from being provided with more statistical information; instead translate concepts into visual aids;
- When presenting uncertainty, use an estimate rather than a range and limit graphs to a single variable.
To help patients make decisions about trial participation, MDIC recommends giving them a decision-making tool that helps them examine their own needs and attitudes and explains trial risks and benefits in terms they can understand.
One example of such a tool, MDIC says, is a template developed by Ottawa Hospital in Canada. The hospital’s Patient Decision Aid (PtDA) helps patients decide between different courses of treatment or between choosing or declining to participate in a trial.
The PtDA lays out information patients need to understand, questions they need to consider – such as what other health factors could affect their choice – and an evaluation of the patient’s priorities that asks such questions as “How important is it to you to avoid side effects” and “How important to you is the benefit of this treatment/trial?”
The template also provides an example of how to explain benefits and risks of the trial in visual form.
The template then presents a checklist that patients can use to gather the key facts they need to make a decision, evaluate how comfortable they are with their decision and what steps to take next (e.g., discuss with my healthcare provider or family).
