The Association of Clinical Research Organizations (ACRO) is urging the FDA to place even more emphasis on risk-based monitoring (RBM) of clinical trials, saying a recent study of its members showed a 16 percent reduction in major findings in audits among sites using that method.
In its comments on the agency’s March draft guidance, ACRO advises the FDA to counter common perceptions of traditional monitoring methods — that they are lower risk, that the strategy of checking every data point is safer and that 100 percent source data verification is the best way of ensuring data quality — by giving RBM the status of “best practice.”
Eighteen additional organizations and individuals — including the Society of Quality Assurance (SQA), Pfizer and Bristol Myers Squibb — weighed in on the guidance in the public comment period that ended May 14.
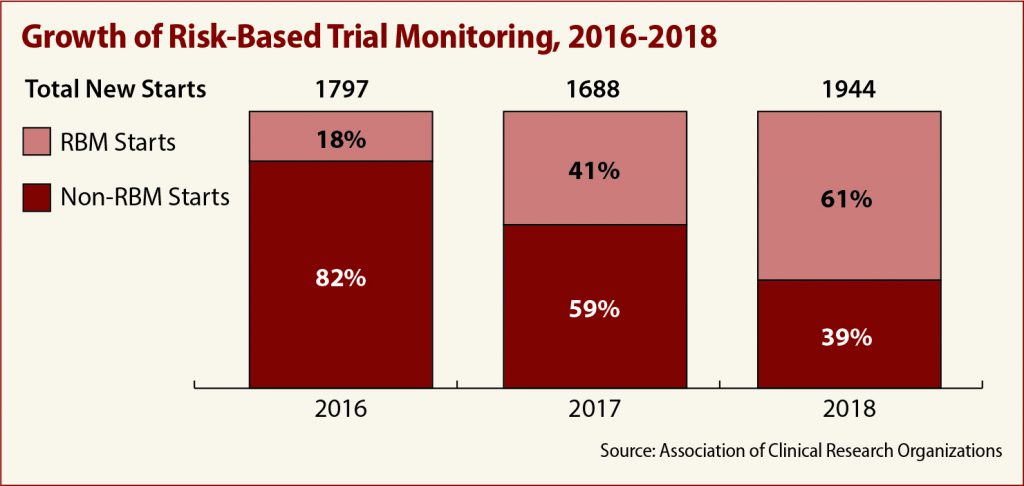
ACRO’s comments include member data showing a marked increase in the use of RBM over the past three years. Only 18 percent of members were involved in trials using RBM in 2016, the association told the FDA. In 2018, that number jumped to 61 percent.
The commitment to RBM appears to be paying off, ACRO reports, in the form of reduced data management time, data entry lag and error rates, as well as cost savings of up to 15 percent.
Findings in the member survey include:
- A 16 percent reduction in critical and major findings in site audits;
- Seventeen percent better detection of significant deviations; and
- Forty-five percent fewer missing pages in RBM trials versus trials using traditional monitoring methods.
But challenges remain, the association says, laying responsibility at the feet of the FDA’s inspection program, which it implies does not recognize the value of RBM. FDA investigators, ACRO told the agency, continue to cite failures in non-critical data results that a trial using risk-based oversight would not address.
Pfizer’s comments on the draft guidance strike the same note regarding inspections. “To encourage robust adoption of risk-based approaches across industry, we suggest that FDA take proactive steps to diminish any perception that an individual inspector may be unwilling to accept this strategy.”
Further, Pfizer would like to know how the FDA intends to measure success of a risk-based approach. “If the Agency intends to measure success through the same parameters of inspection readiness and quality that are used today for non-Risk-Based approach, Pfizer suggests including this language in the guidance.”
ACRO also says it’s unfortunate that the draft document “does not resolve questions within the industry about the definition of central monitoring.”
“Specifically, does centralized monitoring include traditional data cleaning activities, like listings reviews, programmed complex edits, frequencies, etc., in addition to newer, technology-enabled activities, such as statistical analyses, key risk indicators, outlier identification?” the association asks.
Also requesting clarification of the guidance, Bristol Myers Squibb urges the agency to introduce “process-driven categories” of risk-management, noting the draft guidance seems to suggest that “issue management categories” are essential, when, in fact, a good risk management plan focuses on the end-to-end process and teamwork.
SQA’s comments include a recommendation to align the guidance more closely with the international guideline on good clinical practice, ICH E6, and to encourage root cause analysis methods in investigating important deviations.
Read the draft guidance and the full text of all 19 comments here: https://bit.ly/2JHPtYN.
