Dive Brief:
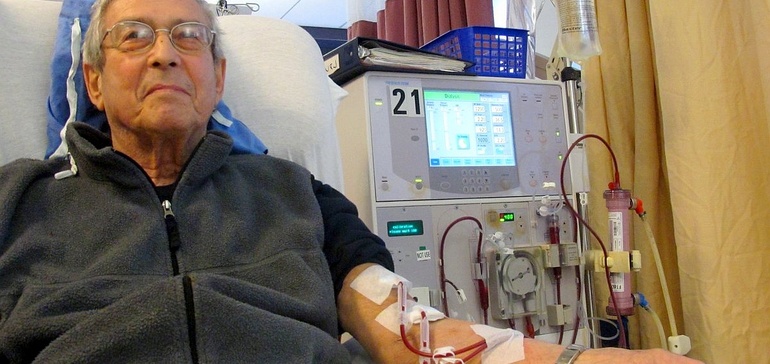
- Fresenius Medical Care received FDA’s breakthrough device designation for a new hemodialysis system, the company said Thursday.
- The system features a polymer called Endexo designed to cut the risk of clotting, thereby reducing the need for physicians to administer blood thinners such as heparin.
- News of the Endexo-enhanced product’s expedited status comes months after Fresenius secured a breakthrough designation for hemodialysis software designed to improve fluid management.
Dive Insight:
Heparin is commonly used during hemodialysis to prevent blood clots. However, use of the drug is associated with a range of near and long-term side effects, including low platelet count, osteoporosis and high cholesterol.
Fresenius, a major provider of dialysis systems, indicated its interest in reducing the risk of these side effects in 2010 when it formed a licensing agreement with Interface Biologics. The agreement gave Fresenius the right to assess the use of Interface’s polymer, Endexo, in its dialysis equipment and an option to license the technology. In 2016, Fresenius extended the license.
Interface designed Endexo to cut platelet adhesion and activation, protein adsorption and thrombus formation, without affecting the mechanical or functional properties of the medical device into which it is incorporated.
Fresenius’ efforts to use the technology in hemodialysis took a step forward in 2018 when it began a clinical trial of equipment featuring Endexo. The study enrolled 26 patients with end-stage renal disease. Initially, participants were treated using Fresenius’ Optiflux hemodialysis machine. Later, they were switched over to equipment that featured Endexo.
The clinical trial wrapped up earlier this year. Fresenius is due to present data from the study next month. In an abstract released ahead of the presentation, Fresenius said patients performed comparably on the established and experimental devices against measures including urea reduction.
In some areas, the Endexo system outperformed Optiflux. The study found the Endexo system was 67% more efficient at removing beta2-microglobulin, a marker that some research suggests predicts mortality.
The breakthrough designation will support Fresenius’ efforts to build on the evidence it has built up over almost a decade working with Endexo to get a device featuring the additive to market. Under the terms of the breakthrough program, Fresenius stands to benefit from closer interactions with FDA and an expedited review.
