What does a site need to do to trim its study start-up timeline from several weeks down to just a few days?
Panelists from various site organizations shared their secrets to rapid start-up and other best practices at MAGI’s “Be the Site of Choice” conference in Philadelphia last week.
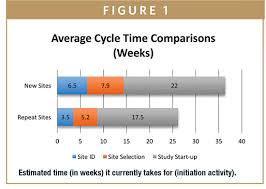
The timelines panelists reported ranged from 45 days all the way down to 12 days, thanks to various time-saving strategies.
At Anne Arundel Medical Center (AAMC) in Maryland, the start-up timeline is 45 days, said Erika Siegrist, research office manager, who credits their success to the ability to reduce the number of committee meetings involved in protocol review. “We really rely on the department level to do the review of the protocols,” Siegrist said, “and we have completely outsourced all of our institutional IRB review.”
AAMC has one institutional committee that meets monthly to review all protocols, she said.
Coming in at 28 days to start-up, VitaLink Research’s founder and vice president of clinical operations Steve Clemons said the fact that the 13-site network’s leadership is located in one place makes things faster. “You can walk across the hall and say, ‘I need you to sign this now,’” Clemons said.
Triad Clinical Trials credits its size for its rapid turnaround. “We’re small,” said president and general manager Scott Whitt. “My coordinators sit within sight of me all day so we can do 12 to 15 days.”
Whitt said he hires very assertive, Type A personalities — usually former charge nurses — as coordinators and holds them accountable from start to finish for everything. “If they can’t kick me around a little bit, I lose respect for them quickly,” he said.
Christina Brennan, vice president of clinical research at Northwell Health, attributed their rapid start-up success to master trial agreements that keep them from reinventing the trial contract wheel. Using a central IRB for all trials they conduct also saves time and eases coordination. But she cautions against sacrificing enrollment for speed. “At the end of the day, if you don’t enroll, you’re not going to be remembered as the site that was the fastest study start-up.”
To achieve enrollment targets, it’s important to “stay in your lane,” Clemons said. “I think it’s truly knowing your patients, knowing your populations, knowing who you have to deal with” that allows your feasibility judgments to be correct.
Clemons said VitaLink’s first patient, first visit timeline is 4.2 days from completion of study start-up.
Whitt’s key to enrollment success is using a clinical trial management system to track all patient data as well as hiring a full-time recruiter who makes 60 to 70 calls a day. “That’s been a real game-changer for us,” he says, because it usually keeps the staff busy screening patients.
All four panelists reported retention rates in the 90 percent range. Siegrist attributes AAMC’s 95 percent rate to their use of dedicated long-term follow-up coordinators to work with patients for 10 to 20 years. The coordinators build a relationship, and patients really look forward to their calls, Siegrist said.
A comfortable environment is Whitt’s key to 92 percent retention. Located in a 1900s-era farmhouse, patients say it’s just like going to grandma’s house. The site offers comfortable couches and big screen televisions for patients, whose visits sometimes last as long as six hours. And because Triad is located in an area with low employment and health insurance coverage, the clinic serves as some patients’ primary care provider.
“We understand our patients at a very deep level,” Whitt said. “We become an important part of their healthcare.”
Whitt also uses what he calls “patient profiles” of various personality types — the accountant, the lawyer, the gossip, the prophet — and thinks in terms of each type’s needs and attitudes. “We more easily identify break points where we might have a protocol that’s not going to do well for us,” he said. “More than likely, we end up finding things that we can do in the clinic to smooth things over.”
Siegrist touted the team-based model for running a successful trial. “I never want to have one coordinator handling everything for a study,” she said. “I always want to make sure there’s a primary and a secondary or pairing a nurse with a non-RN coordinator.”
“We make sure that we have multiple people trained on the same study,” Brennan said, “so everyone can do the same as the primary.”
Whitt builds team motivation by having all the coordinators review feasibility questionnaires before they go to the sponsors. “It creates a sense of ownership and engagement in the study coordinators very early on,” he said.
Clemons said he focuses on matching staff personalities to the tasks they will perform, “learning the personality traits of the people to mix with the patient population that you’re dealing with.” And VitaLink starts early, he said, trying to get people fresh out of school.
“We like to get them and home grow them over the course of a couple of years,” Clemons said, adding that “typically, at two years, if someone’s still with you then usually they’re a decent coordinator.”
By Leslie Ramsey
