Global Blood Therapeutics has seen its sickle cell disease drug approved by the FDA, promising to be the first treatment targeting the root cause of the condition.
The FDA has approved Oxbryta (voxelotor), a daily tablet for the treatment of sickle cell disease in adults and children aged 12 years and older.
GBT said the drug will be available within two weeks and will cost around $125,000 per patient annually.
Oxbryta was approved well ahead of a February 2020 decision deadline, and the company said the approval is a milestone for people living with sickle cell disease.
Until now doctors could only treat the symptoms of the inherited blood disorder that particularly affects people whose ancestors are from sub-Saharan Africa, while Oxbryta tackles the biological mechanism that causes the potentially fatal disease.
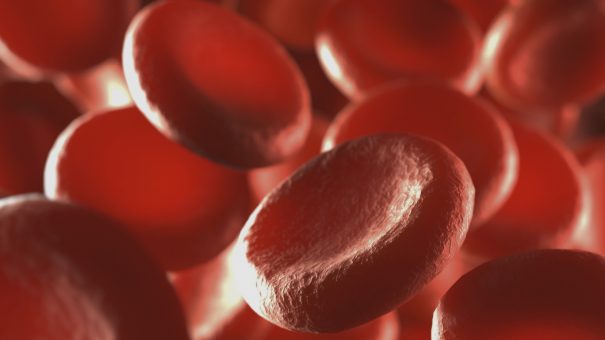
Also affecting people of Hispanic, South Asian, Southern European and Middle Eastern ancestry, the disease causes formation of abnormal haemoglobin.
This abnormal haemoglobin polymerises and causes red blood cells to become sickled, deoxygenated, crescent-shaped and rigid.
This causes anaemia and blocks capillaries and small blood vessels that impede the flow of blood and oxygen in the body.
The resulting damage to tissues and organs can lead to life-threatening complications including stroke and irreversible organ damage.
Oxbryta works by inhibiting the polymerisation process and had been granted a faster six-month priority review after tagging it as a Breakthrough Therapy that could represent a significant improvement over approved treatment options.
The accelerated approval of Oxbryta is based on clinically meaningful and statistically significant improvements in haemoglobin levels, accompanied by reductions in red blood cell destruction.
Data from the phase 3 HOPE study of 274 patients 12 years of age and older with SCD showed that, after 24 weeks of treatment, 51.1% of patients receiving Oxbryta achieved a greater than 1 g/dL increase in haemoglobin compared with 6.5% receiving placebo.
Earlier this month Novartis saw its Adakveo (crizanlizumab) approved to reduce frequency of vaso-occlusive crises in sickle cell disease.
bluebird bio is also working on LentiGlobin, a gene therapy for sickle cell disease which is in the early stages of clinical development.
