Principal investigators continue to have trouble following their own trial plans, an analysis of FDA inspection records shows.
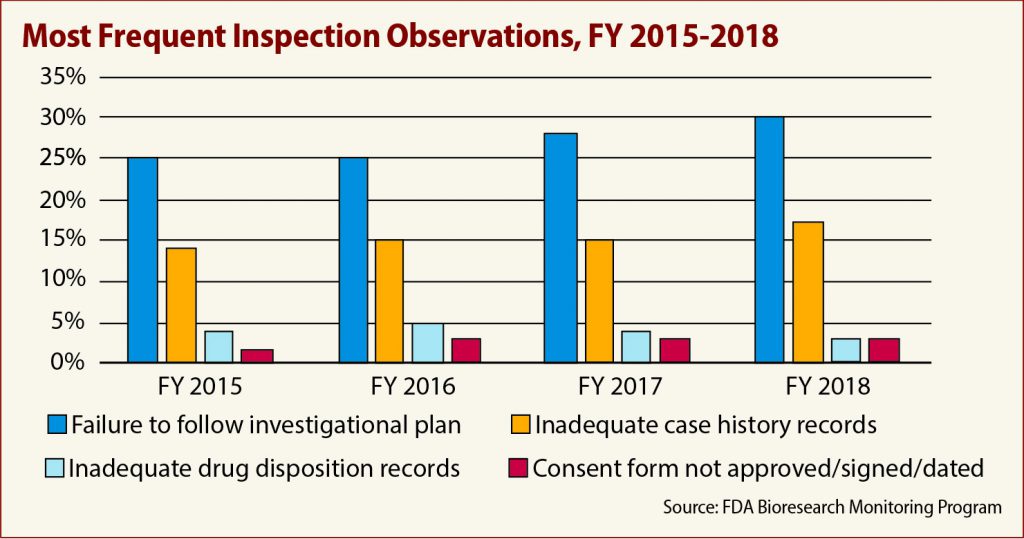
The FDA’s Bioresearch Monitoring Program (BIMO) cited 400 separate failures in fiscal 2018, of which nearly 30 percent — 118 instances — were for failing to follow the investigational plan.
The percentage of protocol violation has been remarkably consistent for several years, data analyzed by CenterWatch show. In fiscal 2017, protocol violations made up nearly 29 percent of all inspection observations. There were more than 25 percent of violations in fiscal 2015 and 2016, respectively.
For Lindsay McNair, chief medical officer for WCG Clinical, the data reinforces three important points. The first is that researchers should remember that there’s no such thing as a protocol waiver.
“There is no mechanism through which prospective planned deviations from the protocol can be granted; these will still be considered failure to follow the investigational plan even if done intentionally,” she says.
The second lesson is that principal investigators must assume total control of those working under them — because they’re certainly going to be given total responsibility for any missteps, McNair says.
Finally, investigators should remember that if an IRB adds new requirements to the study as a condition of approval, then those new conditions are part of the protocol, too, she says.
The second most common citation was for failure to adequately maintain case records — at least 69 separate instances. This, too, seems to have been a consistent problem over time: failure to maintain case records were the second most common problem in fiscal 2015 (92 occurrences), fiscal 2016 (70 times) and fiscal 2017 (76 times).
Other frequent citations include failure to maintain IRB records (14 instances) and failure to maintain proper records of drug doses (13 cases).
BIMO cited five investigators in fiscal year 2018 for conflicts of interest, four for failing to obtain proper informed consent, three for failing to dispose of unused drugs properly, and one for using a site that wasn’t suitably built.
The CenterWatch analysis was based on BIMO’s annual inspection metrics reports from fiscal years 2015 to 2018.
